This article is mainly to help you understand how sealed lead-acid batteries work. First of all, we need to know what sealed lead-acid batteries are.
Invented by French physician Gaston Planté in 1859, sealed lead acid batteries was the first rechargeable battery used for commercial purposes. Despite its age, lead chemistry is still widely used today. Its popularity is well-founded; on a cost-per-watt basis, lead-acid is reliable and cheap. Few other batteries can provide large amounts of electricity as cheaply as lead-acid, which makes batteries cost-effective for cars, golf carts, forklifts, marine and uninterruptible power supplies (UPS).
The grid structure of sealed lead acid batteries is made of lead alloy. Pure lead is too soft to support itself, so a small amount of other metals are added to gain mechanical strength and improve electrical performance. The most common additives are antimony, calcium, tin and selenium. These batteries are commonly referred to as “lead antimony” and “lead calcium”.
Adding antimony and tin can improve deep circulation, but this will increase water consumption and increase equilibrium demand. Calcium reduces self-discharge, but when the positive lead-calcium plate is overcharged, there is a side effect of growth due to grid oxidation. Modern lead-acid batteries also use dopants such as selenium, cadmium, tin and arsenic to reduce the content of antimony and calcium.
Lead acid is heavy and not as durable as nickel-based and lithium-based systems during deep cycling. Full discharge will cause stress, and each discharge/charge cycle will permanently deprive the battery of a small amount of capacity. When the battery is in good operating condition, this loss is small, but once the performance drops to half of the nominal capacity, the attenuation will increase. This wear characteristic is applicable to all batteries to varying degrees.
During deep cycling, lead acid is heavy and not as durable as nickel-based and lithium-based systems. Full discharge will cause stress, and each discharge/charge cycle will permanently deprive the battery of a small amount of capacity. When the battery is in good operating condition, this loss is small, but once the performance drops to half of the nominal capacity, the attenuation will increase. This wear characteristic is applicable to all batteries to varying degrees.
Charging a lead-acid battery is simple, but the correct voltage limit must be observed. Choosing a low voltage limit can protect the battery, but this will result in poor performance and lead to sulfation on the negative plate. High voltage limitation can improve performance, but will cause grid corrosion on the positive plate. If repaired in time, sulfation can be reversed, but the corrosion is permanent.
Lead acid is not suitable for fast charging. For most types, it takes 14-16 hours to fully charge. The battery must always be stored in a fully charged state. Low battery will cause sulfation, which will impair battery performance. Adding carbon to the negative electrode can reduce this problem, but it will reduce the specific energy.
Lead-acid has a moderate lifespan, but it is not as memorable as nickel-based systems, and it has the best charge retention among rechargeable batteries. Although nickel-cadmium batteries lose approximately 40% of their stored energy within three months, lead-acid batteries will self-discharge the same amount within one year. Lead-acid batteries work well at low temperatures and are better than lithium-ion batteries when operating under sub-zero conditions. According to data from the Aachen University of Technology (RWTH) (2018) in Germany, the cost of submerged lead-acid is approximately US$150 per kilowatt hour, which is one of the lowest among batteries.

The first sealed or maintenance-free lead acid appeared in the mid-1970s. Engineers believe that the term “sealed lead-acid” is inappropriate because lead-acid batteries cannot be completely sealed. In order to control exhaust during pressure charging and rapid discharge, a valve is added to release gas if the pressure rises. Instead of immersing the plates in the liquid, but immersing the electrolyte in the wet separator, this design is similar to nickel-based and lithium-based systems. This makes it possible to operate the battery in any physical direction without leakage.
Sealed lead acid batteries contain less electrolyte than flooded batteries, so they are called “acid-deficient”. Perhaps the most significant advantage of sealed lead acid is the ability to combine oxygen and hydrogen to produce water and prevent drying out during the cycle. The recombination occurs at a moderate pressure of 0.14 bar (2psi). If the gas accumulation rises, the valve can be used as a safety vent. Avoid repeated venting, as this will eventually dry out. According to RWTH, Aachen, Germany (2018), the cost of VRLA is approximately US$260 per kWh.
Several types of sealed lead acid have emerged, the most common being gels, also known as valve-regulated lead acid (VRLA) and absorbent glass mats (AGM). The gel battery contains a silica gel type gel, which can suspend the electrolyte in a paste. Smaller packages with a capacity of up to 30Ah are commonly referred to as SLA (sealed lead acid). These batteries are packaged in plastic containers and used for small UPS, emergency lighting and wheelchairs. Due to low prices, reliable services, and low maintenance costs, SLA is still the first choice for healthcare in hospitals and nursing homes. The larger VRLA is used as a backup power source for cellular relay towers, Internet hubs, banks, hospitals, airports, etc.
AGM suspends the electrolyte in a specially designed glass mat. This provides several advantages for lead-acid systems, including faster charging and instant high load currents on demand. AGM is most suitable as a mid-range battery with a capacity of 30 to 100Ah, and is not suitable for large systems such as UPS. Typical uses are the start-up battery of motorcycles, the start-stop function of micro-hybrid vehicles, and ships and RVs that require some riding.
As the cycle and age increase, the capacity of AGM gradually weakens; on the other hand, the gel has a dome-shaped performance curve, staying longer in the high-performance range, but suddenly drops when it is close to the service life. AGM is more expensive than overflow, but cheaper than gel. (The gel is too expensive for starting/stopping the car.)
Unlike submerged lead-acid batteries, sealed lead-acid batteries are designed to have a low overvoltage potential to prevent the battery from reaching its gas-generating potential during the charging process. Overcharging will cause deflation, exhaust, and subsequent water depletion and drying. Therefore, the gel and some AGM cannot be charged to its full potential, and the charging voltage limit must be set lower than the voltage limit for full water. This also applies to floating charge when fully charged. In terms of charging, gel and AGM cannot directly replace the overflow type. If there is no designated charger available for AGM with a lower voltage setting, please disconnect the charger after 24 hours of charging. This can prevent the generation of gas due to the setting of an excessively high float voltage.
The best operating temperature for VRLA batteries is 25°C (77°F); an increase of 8°C (15°F) above this temperature threshold reduces battery life by half. The rated discharge time of lead-acid batteries is 5 hours (0.2C) and 20 hours (0.05C). The battery performs best when discharged slowly; the capacity reading is much higher when discharged at a slower rate compared to the 1C rate. However, if only used for a few seconds, lead acid can provide a high pulse current of several C. This makes lead acid very suitable as a starter battery, also known as starter light ignition (SLI). The high lead content and sulfuric acid make lead acid unfriendly to the environment.
Lead acid batteries are commonly classified into three usages: Automotive (starter or SLI), motive power (traction or deep cycle) and stationary (UPS).
The starter battery is designed to start the engine with an instantaneous high-power load that lasts about one second. In terms of its size, the battery can provide high current, but it cannot perform deep cycles. The starter battery is rated Ah or RS (reserve capacity) to indicate energy storage capacity, and CCA (cold start amplifier) to indicate the current that the battery can provide at low temperatures. SAE J537 stipulates that the battery is discharged at the rated CCA ampere for 30 seconds at –18°C (0°F), and the battery voltage will not drop below 7.2 volts. RC reflects the running time at 25 minutes of stable discharge. (SAE stands for Society of Automotive Engineers.)
The starting battery has a very low internal resistance, which is achieved by adding additional plates to obtain the maximum surface area (Figure 1). The board is very thin, the lead is applied in a sponge-like form, and the appearance is fine foam, which further expands the surface area. The plate thickness, which is important for deep cycle batteries, is not so important because the discharge time is short and the battery is charge while driving; the focus is on power rather than ability.
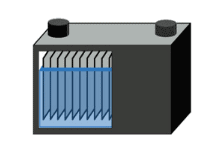 | Figure 1: Starter battery The starter battery has many thin plates in parallel to achieve low resistance with high surface area. The starter battery does not allow deep cycling. Courtesy of Cadex |
Deep-cycle batteries are designed to provide continuous power for wheelchairs, golf carts, forklifts, etc. The battery is designed for maximum capacity and reasonably high cycle times. This is achieved by thickening the lead plate (Figure 2). Although the battery is designed for cycling, full discharge still causes stress, and the number of cycles is related to the depth of discharge (DoD). Deep cycle batteries are marked in Ah or minutes of running time. Usually the rated capacity is 5 hours and 20 hours discharge.
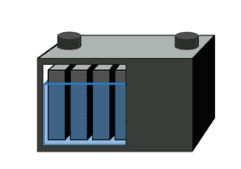 | Figure 2: Deep-cycle battery The deep-cycle battery has thick plates for improved cycling abilities. The deep-cycle battery generally allows about 300 cycles. Courtesy of Cadex |
Starter batteries cannot be interchanged with deep cycle batteries, and vice versa. Although creative elderly people may install a starter battery in a wheelchair instead of the more expensive deep cycle to save money, the starter battery will not last because the thin sponge-like plate will quickly dissolve as the deep cycle is repeated.
There are combination starter/deep cycle batteries that can be used in trucks, buses, public safety, and military vehicles, but these units are large and heavy. As a simple guideline, the heavier the battery, the more lead it contains and the longer its service life. Table 3 compares the typical life of the starter battery and the deep cycle battery during deep cycle.
| Depth of discharge | Starter battery | Deep-cycle battery |
| 100% 50% 30% | 12–15 cycles 100–120 cycles 130–150 cycles | 150–200 cycles 400–500 cycles 1,000 and more cycles |
Table 3: Cycle performance of starter and deep-cycle batteries. A discharge of 100% refers to a full discharge; 50% is half and 30% is a moderate discharge with 70% remaining.
The work of lead-acid batteries is entirely related to chemistry, and it is very interesting to learn about it. The charge and discharge state of lead-acid batteries involves a large number of chemical processes. When the acid dissolves, the dilute sulfuric acid H2SO4 molecule will split into two parts. It will produce positive ion 2H+ and negative ion SO4-. As we said before, the two electrodes are connected as a plate, the anode and the cathode. The anode captures negative ions, and the cathode attracts positive ions. This bonding in the anode and SO4- and cathode exchanges electrons with 2H+, and further reacts with H2O or with water (dilute sulfuric acid, sulfuric acid + water).
The battery has two states of chemical reaction, Charging and Discharging.
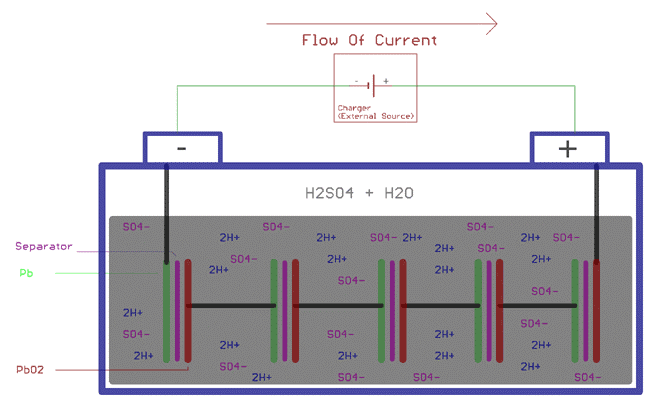
As we know, to charge a battery, we need to provide a voltage greater than the terminal voltage. So to charge a 12.6V battery, 13V can be applied.
But what actually happen when we charge a Lead Acid Battery?
Well, the same chemical reaction as we described earlier. Specifically, when the battery is connected to the charger, the sulfuric acid molecule will decompose into two ions, the positive ion 2H+ and the negative ion SO4-. The hydrogen exchanges electrons with the cathode and becomes hydrogen, which reacts with PbSO4 in the cathode to form sulfuric acid (H2SO4) and lead (Pb). On the other hand, SO4- exchanges electrons with the anode to become radical SO4. This SO4 reacts with PbSO4 at the anode and produces lead peroxide PbO2 and sulfuric acid (H2SO4). It stores energy by increasing the gravity of sulfuric acid and increasing the battery potential voltage.
As explained above, following chemical reactions takes place at Anode and Cathode during the charging process.
At cathode
PbSO4 + 2e- => Pb + SO42-
At anode
PbSO4 + 2H2O => PbO2 + SO42- + 4H- + 2e-
Combining above two equation, the overall chemical reaction will be
2PbSO4 + 2H2O => PbO2 + Pb + 2H2SO4
There are a variety of methods suitable for charging lead-acid batteries. Each method can be used for specific lead-acid batteries for specific applications. Some applications use a constant voltage charging method, some applications use a constant current method, and tickling charging is also useful in some situations. Usually the battery manufacturer will provide the correct way to charge a specific lead-acid battery. Constant current charging is usually not used for lead-acid battery charging.
The most commonly used charging method for lead-acid batteries is the constant voltage charging method, which is an effective charging time process. During the full charge cycle, the charge voltage remains constant, and the current gradually decreases as the battery charge level increases.
The discharge of lead-acid batteries again involves chemical reactions. Sulfuric acid is in diluted form, usually in a 3:1 ratio to water and sulfuric acid. When the load is connected across the board, the sulfuric acid is again decomposed into positive ions 2H+ and negative ions SO4. Hydrogen ions react with PbO2 to produce PbO and water H2O. PbO starts to react with H2SO4 and produce PbSO4 and H2O.
On the other hand, SO4- ions exchange electrons with lead to produce free radical SO4, which further produces PbSO4 which reacts with lead.
As mentioned above, during the discharge process, the anode and cathode will undergo the following chemical reactions. These reactions are completely opposite to the charging reactions:
At cathode
Pb + SO42- => PbSO4 + 2e-
At anode
PbO2 + SO42- + 4H- + 2e- => PbSO4 + 2H2O
Combining above two equation, the overall chemical reaction will be
PbO2 + Pb + 2H2SO4 => 2PbSO4 + 2H2O
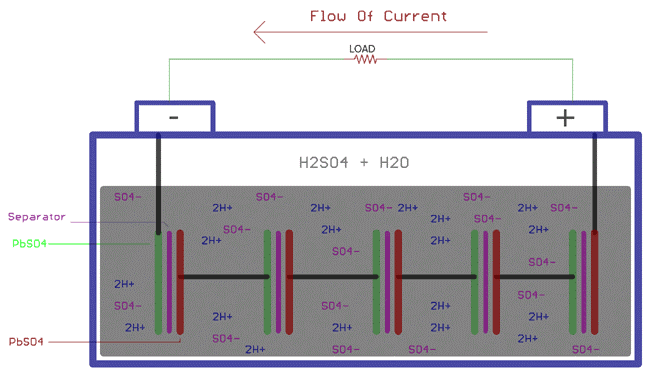
Due to the electronic exchange between the anode and the cathode, the electronic balance between the plates is affected. Then electrons flow through the load and the battery discharges.
During this discharge process, the proportion of dilute sulfuric acid drops. Moreover, at the same time, the potential difference of the sealed lead acid battery decreases.
Bu understanding the working principle of sealed lead-acid batteries, we can know that the new substance produced by the chemical reaction of lead-acid batteries is water. Compared with dangerous batteries such as lithium batteries, lead-acid batteries are safer and more environmentally friendly.