Lead acid batteries are chemical energy storage devices which are applied in various occasion. Lead acid battery types are divided depends on the functions and material, but the chemical principle is still similar which is using lead and lead dioxide as the negative and positive active materials of the battery and dilute sulfuric acid as the electrolyte. It has high power conversion efficiency, long cycle life, high terminal voltage, high safety, and high-cost performance with simple installation and maintenance features. At present, it is the preferred chemical power source among all kinds of energy storage, emergency power supply, and start-up devices.
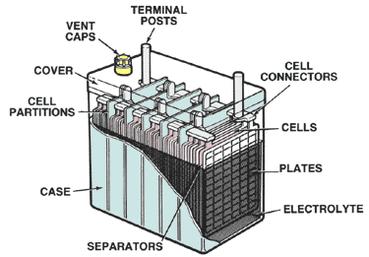
The main components of lead-acid batteries include plates, separators, electrolytes, and battery cover.
1. Polar plates: Both the positive and negative electrodes are made of special alloy grids which are coated with active materials. The plates store and release electricity during charging and discharging to ensure the reliability of the battery’s capacity and performance.
2. Separator: A separator is an isolation medium placed between the positive and negative electrodes of the battery to prevent the battery’s positive and negative poles from directly contacting and short-circuiting. Different types of lead-acid batteries have different separator materials. Valve-regulated batteries use AGM/PE/PVC mainly.
3. Electrolyte: The electrolyte of a lead-acid battery is dilute sulfuric acid prepared with distilled water. The electrolyte plays a role in transmitting ions between the positive and negative electrodes during charge and discharge. Therefore, the electrolyte must be free of impurities.
4. Container (battery case cover): The electrolyte and the electrode plate are in the container. The container mainly plays a supporting role, and at the same time prevents internal materials from spilling or external materials from entering the internal structure to pollute the battery.
The working principle of a lead-acid battery is to convert electrical energy and chemical energy through an electrochemical reaction. The electrode is mainly made of lead and its oxide, and the electrolyte is a kind of storage battery with a sulfuric acid solution. There are many types of lead-acid batteries, and two of them are used in photovoltaic battery systems. The two lead acid battery types are flooded lead-acid batteries and valve-regulated sealed lead-acid batteries.

LEOPARD AGM battery series
In a traditional lead-acid battery, the remaining space in the battery tank except for the plates, separators, and other assembly parts is filled with sulfuric acid electrolyte. The board is completely immersed in the sulfuric acid electrolyte.
During use, due to the evaporation and decomposition loss of water, it is necessary to open the cover regularly to add distilled water and adjust the electrolyte density. So it is customary to lead Acid batteries are also called “open-type” batteries.
Valve-regulated sealed lead-acid batteries, also known as maintenance-free batteries, are divided into AGM sealed lead-acid batteries and GEL gel-sealed batteries.
AGM battery uses pure sulfuric acid aqueous solution as an electrolyte, most of the electrolyte exists in the glass fiber membrane, and there is a part of the electrolyte inside the electrode plate. The AGM sealed lead-acid battery has a small amount of electrolyte, a thicker plate, and a lower active material utilization rate than an open-type battery, so the battery’s discharge capacity is about 10% lower than it.

LEOPARD gel battery series
Compared with gel-sealed batteries, AGM sealed lead-acid batteries have a smaller discharge capacity and are more expensive than flooded batteries of the same specification. However, AGM batteries have the following advantages:
(1) The cyclic charging capacity is 3 times higher than that of lead-calcium batteries and has a longer service life.
(2) Having higher capacitance stability throughout the life cycle.
(3) Low-temperature performance is more reliable.
(4) Low accident risk and environmental pollution risk
(5) The maintenance is simple and the deep discharge is reduced.
The advantages of gel-sealed lead-acid batteries are as follows:
(1) The probability of acid leakage is small. There is no free electrolyte after the electrolyte gel in the GEL battery because the probability of acid leakage is much smaller than that of the AGM battery.
(2) Less water loss. Because it has more perfusion than dilute sulfuric acid and less water loss, the GEL battery will not fail due to water loss.
(3) Effectively extend battery life. The infusion of colloid increases the strength of the separator, protects the polar plates, makes up for the defect of the separator contracting with acid, and makes the assembly pressure not significantly reduced, which is one of the reasons for its prolonged battery life.
(4) The gel battery has strong resistance to overcharge.
(5) Strong recovery ability under severe discharge conditions.
In general, the over-discharge recovery capability and low-temperature charge-discharge performance of GEL batteries are superior to those of AGM batteries.
AGM batteries and gel batteries are two sealed lead-acid battery types manufactured using different process technologies. They have big differences in design ideas, fixing methods of electrolyte, assembly requirements, and battery structure and performance. For AGM batteries, AGM separators are used to fix the electrolyte. Generally, lean electrolyte design and tight assembly structure are adopted to make the battery lighter and have better high-current discharge performance, which is more suitable for UPS applications with shorter expected service life. occasion. For the gel battery, it adopts the flooded design, the battery has good deep discharge performance, good stability and reliability, low self-discharge, long shelf life, less thermal runaway, and is more suitable for applications with a long-expected service life occasion.